Lead Program - EF-210
Indication: Myotonic Dystrophy Type 1 (DM1)
DM1 is a multisystem disorder effecting skeletal muscle, eye, heart, CNS, endocrine systems, and the most prevalent triplet repeat disease. 1 in 8,000 ~ 20,000 people are afflicted with DM1 worldwide, with estimated 15,000 patients in Japan and 150,000 in Japan, US, and Europe combined. Unfortunately, there is currently no FDA-approved treatment and patients with DM1 suffer from severe symptoms and face high unmet medical needs.
DM1 is caused by an increase in the number of CUG triplet repeats found in the myotonic dystrophy protein kinase (DMPK) gene. While the number of CUG units within the 3' UTR of DMPK mRNA ranges 5 to 37 in normal subjects, more than 50 (CUG) units are observed In DM1 patients. The over-extended CUG repeats forms the hairpin structure which captures/binds up MBNL, the protein playing essential roles in RNA splicing, causing abnormal muscle function.
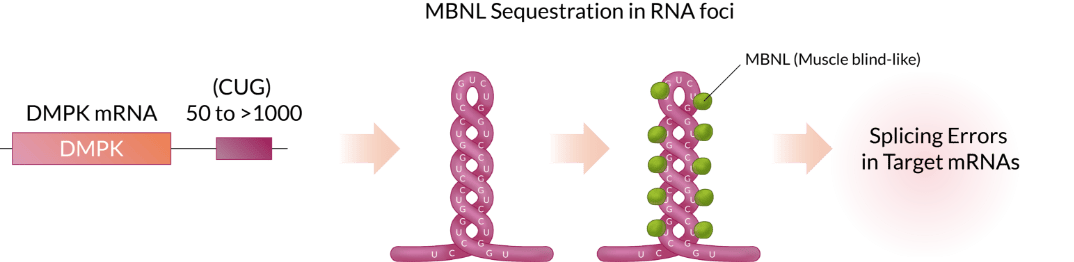
Approach
EF-210 is a recombinant AAV that delivers a therapeutic molecule into patients’ muscles. The therapeutic molecule CUG-PPR1 specifically binds to pathogenic RNA, thereby blocking the binding between MBNL and CUG RNA repeat. This increases the levels of functional free MBNL proteins and reduces RNA foci, to restore normal splicing activity and muscle function.
Our research paper on efficacy of EF-210 was published in Science Translational Medicine. Please see here for details.
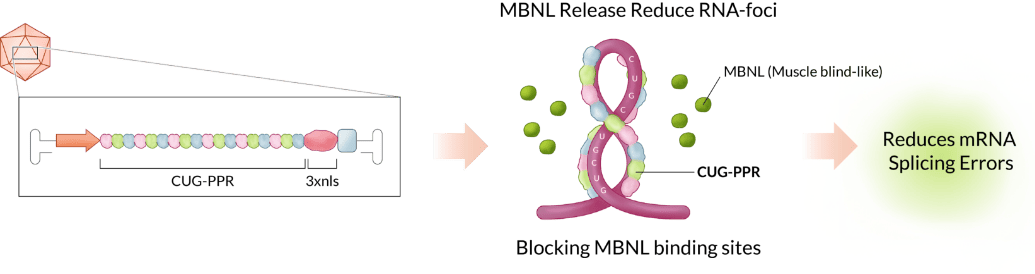
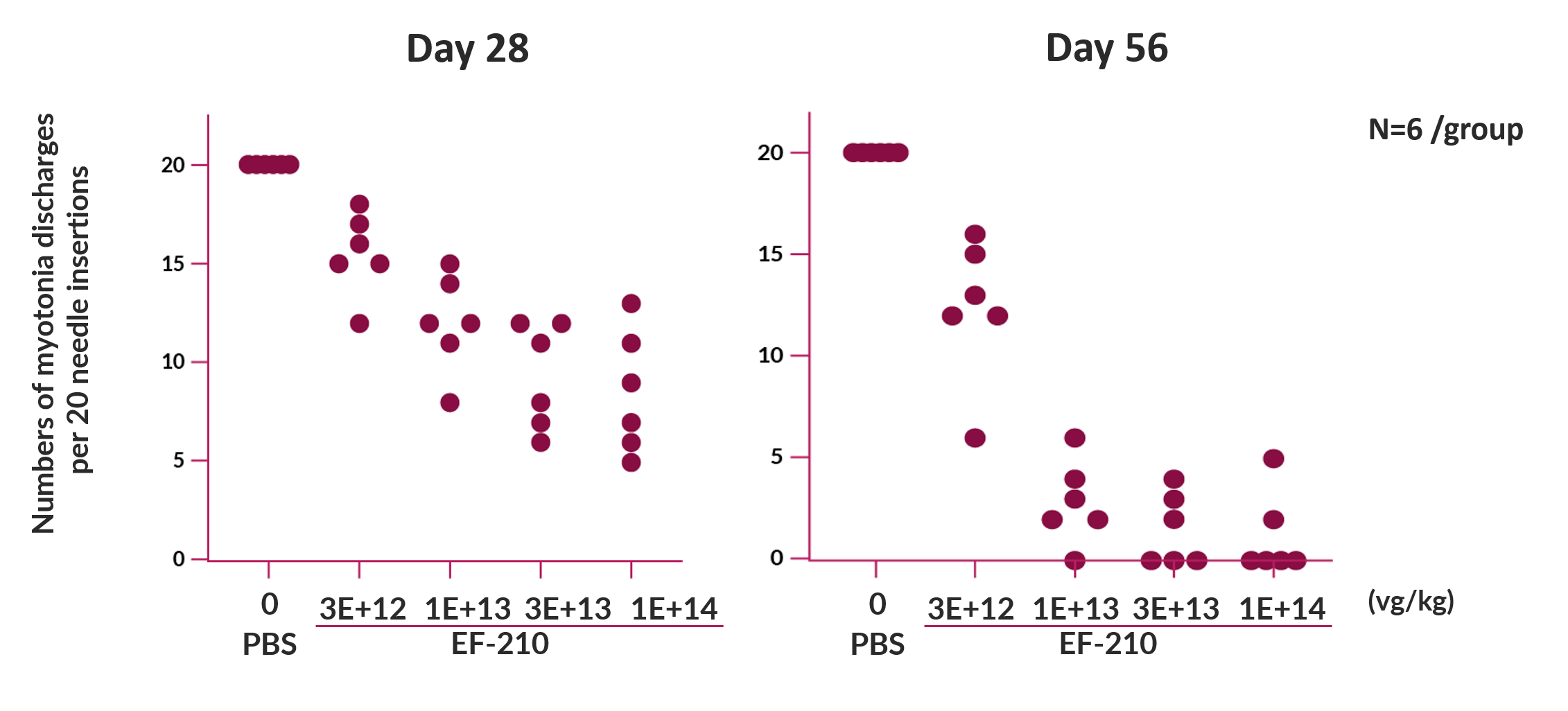
Proof of Concept
In a in vivo pharmacology study, the EF-210 drug substance was administered once IV to groups of HSALR mice. In this study, the maximum effect, where no myotonia events were observed in any of 20 needle insertions, was noted in some mice at 1 × 1013 vg/kg and higher doses on Day 56.
Development Plan
- Proof of Concept (POC) study was completed in DM1 mouse model.
- Non-GLP studies in both mice and NHPs have been completed.
- IND-enabling GLP study is ongoing.
- submission and first-in-human trial are scheduled in early 2027.
